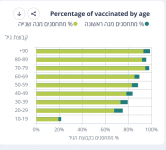
Here doser. You want numbers-:
By mid March, 2021, vaccination against COVID-19 using the ChAdOx1 nCoV-19 (AZD1222) vaccine from Oxford–AstraZeneca1,2 was paused in a number of European countries due to reports of thromboembolic events in vaccinated individuals.3 According to the European Medicines Agency (EMA), 30 cases of...
www.thelancet.com
This is Astra Zeneca.
"According to the European Medicines Agency (EMA), 30 cases of thromboembolic events (predominantly venous) had been reported by March 10, 2021, among the approximately 5 million recipients of the Oxford–AstraZeneca COVID-19 vaccine in the European Economic Area."
"In a population of 5 million people (ie, size matching the approximate number of people having received the Oxford–AstraZeneca COVID-19 vaccine in Europe by March 10, 2021), this incidence would correspond to approximately 169 expected cases of venous thromboembolism per week, or 736 expected cases per month (if based on the incidence rate among the 18–99-year-old Danes). Similarly, if estimated based on the incidence rate among 18–64-year-old Danes, one would expect 91 cases of venous thromboembolism per week, or 398 cases per month."
"Here, based on pre-pandemic incidence rates from the entire Danish population, we report that the number of venous thromboembolisms reported in relation to the Oxford–AstraZeneca COVID-19 vaccine does not seem to be increased beyond the expected incidence rate."
You can read the rest, and the appendix, yourself.
In the US, there have been 6 cases of thrombosis out of 7 million vaccinations with the J&J vaccine. I have not seen a peer reviewed paper on thsi yet, but you do the math.