They use supercritical water to drill into rocks under water.
Yes, they do.
And what happens to it after it leaves the nozzle? It quickly becomes room temperature, and is no longer supercritical.
It cooled off.
When this hot supercritical water leaves the nozzle it does not cool.
Denying reality isn't healthy, Vowels.
Hot supercritical water does in fact cool when released from it's container.
Just like compressed air cools when it leaves the can.
Just like butane does when it leaves the can.
Just like water vapor does when it leaves the kettle.
This is a known fact of physics. When fluids expand, they cool, RAPIDLY.
That's why frost forms on the compressed air can and butane refill. That's why steam only a short distance from the whistler is cool, while the water inside the kettle is still boiling hot.
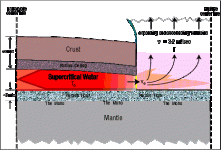
The same applies to the fountains of the great deep. A mile deep chamber of supercritical fluids (I say fluids because it wasn't just water, but minerals as well, and this is important), and its container ruptured, causing the fluids contained within to expand through the now 60 mile deep crack, forcing it to get wider and wider, literally ripping the crust apart like the seam on a baseball in the space of about 2 hours. If you have a fluid, even if it's in a supercritical state, expanding for 60 miles, it cools, RAPIDLY, to the point where the waters were so cold, they would have been frozen, if it weren't for the extremely high mineral content which lowered it's freezing point to well below what the temperature ended up as.
And because it's being launched straight upwards (directed energy), it doesn't just "expand outwards" easily, and is continued to be pushed upwards by the fluids beneath it, also being launched upwards, with enough energy to carry it into space, where it cools even further. Some of it falls back to earth, the rest of it becomes part of the debris we see scattered around the solar system.
This expanding fluid also erodes away the 60 mile tall cliff walls as they collapse (cliffs taller than 5 miles will collapse under their own weight, shearing off until it reaches a stable point). The debris from this would have been launched upwards along with the still expanding supercritical (though, not supercritical by the time it reaches the top of the crust.
In fact the problem is it entrains or drags with it the cold water, thus cooling the cutting water and rendering it less effective.
See Stripe's comment above.
So the answer in practice to all your above questions is that supercritical water in a watery environment stays hot unless the surrounding water cools it before it hits the rock it is meant to cut.
What makes you think I'm talking about the water in the chamber?
I've been talking about the fountains (and have said as much) for the past several posts.
What part of "the fountains of the great deep were cold" do you not understand?
There is no complaint that the water leaving the nozzle cools.
That's what I've been talking about, you nincompoop.
Maybe this will help you understand:
See
https://pangea.stanford.edu/ERE/pdf/IGAstandard/SGW/2012/Schuler.pdf
I read the creation.com site which said the supercritical water cools,
Maybe instead of creation.com, you should go to hpt.rsr.org/onlinebook/ and read from there.
They're right. Supercritical water does cool. But only when it expands.
and can be seen in the frozen dinosaurs. Does not.
Does not what? Cool? Yes, it does, when it expands.
Yes, and?
The steam at atmospheric pressure has the same temp of 100C.
But it doesn't stay at that temperature unless it's contained.
Because fluids cool when they expand.
The fountains of the great deep went from supercritical to subzero as they expanded through the crack in the crust, and continued expanding far into the atmosphere.
And the butane cools as it leaves the can because it is changing phase from liquid to gas.
And supercritical fluids cool as they leave the subterannean chamber because it is changing phase from supercritical to gaseous liquid to nearly frozen slush with an extremely high mineral content.
Supercritical water is already hotter than 100C at 400C so it has lots of energy to draw on.
Correct. See the image above.
And it is already in a partially gas phase, so it may already have absorbed latent heat and so would not cool when it leaves the nozzle.
So you're saying that a hot fluid will not cool when it expands?
You're disagreeing with the laws of physics at this point, so there's not much more I can say.
Like I said practical experience with cutting with supercritical water show the water comes out super hot, not cold.
As Stripe said, they intentionally design the nozzles so that the water is as hot as possible when it comes out.
The cracks in the crust of the earth were not designed that way, or at all, for that matter, and would have gotten wider and wider as time went on, allowing for more and more expansion of the fluids being released.
Facts don't care about your feelings.
but then we are left with a frozen earth.
What?
But the energy in a closed system remains the same. Mix hot and cold water in equal proportions and they combined reach the temp in between of 210C. That is the real science. What happens at the nozzle is local and irrelevant.
See the above image.
But there is no way the released stream does not expand. So why is it not hot where it cuts and frozen all around.
What are you even talking about at this point?
Water at Ocean Vents Isn't Water—It's a Gas-Liquid Hybrid
You've heard about the freaky animals at ocean vents. Now check out the freaky water.
In the physical world, it sometimes seems that rules are made to be broken. Even the simplest principles can’t be taken at face value. Take water. A few molecules of H2O at normal atmospheric pressure are solid when below 32 degrees Fahrenheit, liquid between 32°F and 212°F, and gas when above 212°F. But this past August, when scientists reported observing water from a hydrothermal vent acting like a liquid and a gas simultaneously at 867°F, nature had thrown them for a loop. This was the first measurement of a phase transition–defying supercritical fluid.
It turns out the standard line about matter existing in three states—solid, liquid, and gas—is only part of the story. In extreme conditions—in this case, magma-heated water at an ocean depth of nearly 10,000 feet—things work a little differently. Any increase in temperature or pressure beyond a particular “critical point” (see the chart below) puts a material into the supercritical zone, “where the distinction between liquid and gas disappears,” says Virginia Tech polymer scientist Erdogan Kiran, who studies supercritical fluids.
You've heard about the freaky animals at ocean vents. Now check out the freaky water.

www.discovermagazine.com
Notice how supercritical water is ejected from ocean vents at 857F (458C). That is hot, not cold. And the water has expanded, causing heating not cooling.
It's still in the process of expanding. That's why it's being ejected through those vents.
You seem to be avoiding the point we're making, Vowels.
The point being that extremely hot pressurized fluids being released causes them to become cold, and if they have nowhere to go but upwards through the crust from their subterannean chamber, they will continue expanding until they reach equilibrium, both in pressure and temperature.
In other words...
There are at least twelve different factors that you haven't even come close to addressing that reduce heat:
Twelve Factors Help to Answer Flood Heat Objections: This list provides a roadmap for the listener to know where we will be headed over the next few episodes as we evaluate the heat consequences of the hydroplate theory. (Italics indicates material added after the series ended.)
1. Fluids cool rapidly as they expand (as in from below the crust to the surface) as well described by the Joule-Thomson effect.
2. Directed energy comprised of molecules with great momentum strongly resists change in direction.
3. Boundary conditions, rather than total amount of heat, determine how much will transfer, e.g., to the atmosphere or ocean.
4. Water that is supercritical (its state in the subterranean chamber, and unlike liquid water at Earth's surface) is highly compressible and at sixty miles deep it was compressed by pressure greater than 370,000 lbs per square inch.
5. Understanding the behavior of supercritical water helps to quantify the heat of the fountains including that as it enormously expands to reach the 15 ppsi at Earth's surface the formerly SCW has cooled tremendously according to the slope defined by the Clausius-Clapeyron relation.
6. Outer space functions as a virtually infinite heat "sink" radiating away (cooling) the fountains most energetic water and debris (including much of the heat generated by friction as many water molecules and some debris falls back through Earth's atmosphere); as most of the large (and sometimes hot) solids were ejected into space.
7. Air is a great insulator [like home insulation and Thinsulate].
8. Z-pinch (crustal lightning making heavier nuclei including dangerous radioactive elements like uranium and thorium) is adiabatic (i.e, it doesn't produce heat) and is even called cold repacking.
9. Time, even the duration of weeks and months (or years and even a few centuries of aftermath effects), can allow for the dissipation of large quantities of energy that would otherwise melt more of the Earth than actually did melt.
10. Estimates provided by critics trying to falsify the hydroplate theory can be shown to stop suddenly short of affirming the hydroplate.
11. Forty days and nights (especially the nights) of torrential rain brought massive quantities of supercooled hail down onto the Earth.
12. The specific heat of water (i.e., a watched pot never boils), also called its heat capacity, is higher than any other common substance enabling the surface waters to absorb a tremendous amount of energy while raising its temperature minimally.
13. Greater albedo (reflectivity) of the Earth from increased cloud cover would have significantly reduced incoming solar energy (and reflected away heat radiating earthward from debris falling through the upper atmosphere). |

rsr.org